Status COVID-19/Flu A&B
For Professional Use Only
Quantity: 25 Tests/Kit
Sample Type: Nasopharyngeal
Storage Temperature: 2-30°C
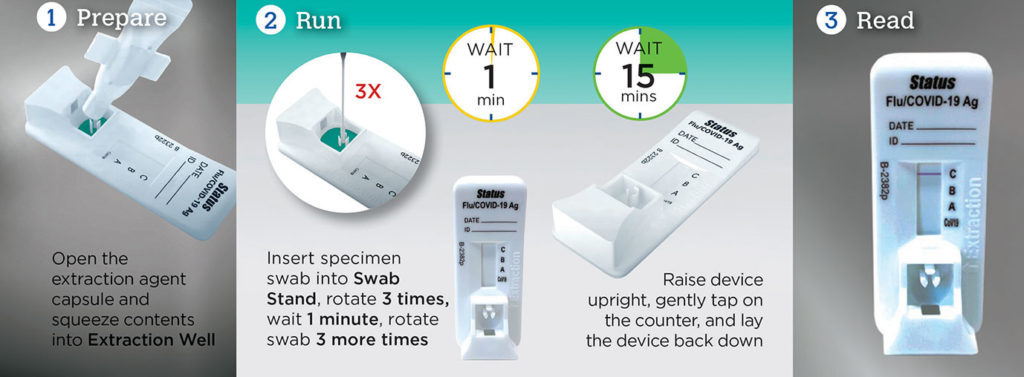
Test Time: 15 minutes (Do not read results after 20 minutes)
Each Kit Contains 25 Tests, $23 each ($575 per Kit). In Stock. Order Today!
If you’re looking for 10,000 tests or more, please contact us:

A Rapid Immunoassay for the Simultaneous Direct Detection and Differential Diagnosis of SARS-CoV-2, Influenza Type A and Type B Antigen from anterior nasal and nasopharyngeal swab specimens.

Benefits of using Status™ COVID-19/Flu A&B
COVID-19 - Anterior Nasal - Sensitivity 93.8%, Specificity 100% Nasopharyngeal - Sensitivity 93.1%, Specificity 100%
Flu A - Sensitivity 91.4%, Specificity 95.7%
FDA Emergency Use Authorization (EUA)
Flu B - Sensitivity 87.6%, Specificity 95.9%
Visually read in 15 minutes
Flocked swabs for superior specimen collection and patient comfort
Customer Testimonials
I’ve known the people at Northern Diagnostics for over 30 years, working with them on research and development and as a customer. They are a fantastic team who I plan on working with for many more years to come.

After learning about Lumos Diagnostics' FebriDx test through Northern Diagnostics and utilizing this point of care test myself, I am excited to witness the impact that a diagnostic test differentiating viral v bacterial infection in 10 minutes will have on the Canadian population. Rapid diagnostic tests, such as FebriDx, should be tried by our healthcare community for the speed, accuracy and value they provide to Canadians by minimizing the overuse of antibiotic prescriptions.

I couldn’t be happier with the service I received from Northern Diagnostics! Friendly, knowledgeable staff who offer prompt and professional services and high quality equipment at unbeatable prices.

I recently purchased a portable ultrasound machine to be more flexible. My new DP-30 is top quality (as expected) and makes it easy for me to see my patients with thyroid disorders at different clinics around the city. In short: I’m very happy and satisfied with the product and the personalized service.

I wanted the best image quality I could get for my money, and at this point in my career, I wanted an ultrasound I enjoyed using. With the DP-30 I purchased from Northern Diagnostics, I felt like I got great value and a system I really enjoy using. Northern Diagnostics made me feel like they cared about my experience when I use my ultrasound, and that is important to me.

Previous
Next